- Progression Free Survival of 7 months after Treatment with KiroVAX/BSK01 and Chemotherapy Versus 3.9 months with Standard of Care
- KiroVax/BSK01 Underscores the Company’s Cellular Therapy Approach to Treating Solid Cancer Tumors
- Advancement of KiroVax/BSK01 Leverages Acceleration of Regulatory Pathways, Improvements in Manufacturing Yield, and Potential as an Adjuvant Treatment to Improve Efficacy of Company’s Broader Pipeline
Kiromic BioPharma, Inc. (NASDAQ: KRBP) (“Kiromic” or the “Company”), a clinical-stage fully integrated biotherapeutics company using its proprietary DIAMOND® artificial intelligence (AI) platform to discover and develop cell and gene therapies with a therapeutic focus on immuno-oncology and other diseases, today announces the results of a published pilot Phase 1 clinical trial that showed KiroVax/BSK01, Kiromic’s cell therapy cancer vaccine candidate, in combination with chemotherapy, demonstrated a significant progression free survival (PFS) benefit in one of the patients with metastatic pancreatic cancer who participated in the trial.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20211025005273/en/
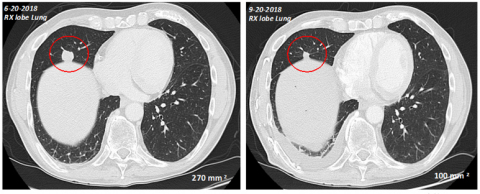
Figure 1: stable disease in a pancreatic cancer patient with lung metastasis which initially increased in size to 270mm2 and then subsequently stabilized at 100mm2 after three months of treatment with KiroVax/BSK01 in combination with chemotherapy (Photo: Business Wire)
The results from the pilot Phase 1 clinical trial demonstrated PFS of 7 months after treatment with KiroVAX/BSK01 and chemotherapy versus PFS of 3.9 months with second line chemotherapy.
“We are very proud of the results we achieved with KiroVax/BSK01 in addressing advanced metastatic pancreatic cancer. We believe that this is an exciting study, as we believe it demonstrates Kiromic’s early clinical success in the cellular therapy arena,” stated Maurizio Chiriva Internati, DBSc, PhDs, President, Chief Executive Officer, Chairman, and Founder of Kiromic BioPharma. “By reaffirming this published clinical study we intend to illustrate Kiromic’s robust cellular therapy platform, and accordingly, we believe that KiroVAX/BSK01 may have a powerful and synergistic impact as an adjuvant to our novel and innate immunity cellular therapy pipeline. As we did with KiroVax/BSK01, we expect to be able to also progress Kiromic’s CAR-T therapies to the clinical arena.”
Metastasis is the spread of cancer cells from the place where they first formed to another part of the body. In metastasis, cancer cells break away from the original (primary) tumor, travel through the blood or lymph system, and form a new tumor in other organs or tissues of the body. The new, metastatic tumor is the same type of cancer as the primary tumor.
The scans in figure 1 illustrate stable disease in a pancreatic cancer patient with lung metastasis which initially increased in size to 270mm2 and then subsequently stabilized at 100mm2 after three months of treatment with KiroVax/BSK01 in combination with chemotherapy, resulting in a progression free survival (PFS) of 7 months.
More information on this Phase 1 clinical trial may be found on the Company’s website.
Based on these encouraging results, the Company intends to evaluate the potential role of KiroVax/BSK01 as an adjuvant in combination with its growing innate immunity cellular therapy platform. Kiromic added to its innate immunotherapy portfolio by also developing multi-indication, Gamma Delta T-cells, which have the added commercial advantage of being “off-the-shelf” cellular therapeutic candidates to address solid tumor cancers.
About KiroVax/BSK01
KiroVax/BSK01, the Company’s Phase 1 cell therapy cancer vaccine candidate, consists of professional antigen presenting cells that are matured and pulsed with tumor specific antigens, yielding a tumor-targeted, next-generation cell therapy vaccine designed for the therapeutic treatment of multiple types of solid cancer tumors. The Company plans on progressing KiroVax/BSK01 into subsequent clinical trials.
About Kiromic BioPharma
Kiromic BioPharma, Inc. is a clinical-stage, fully integrated biotherapeutics company using its proprietary DIAMOND® artificial intelligence (AI) platform to discover and develop cell and gene therapies with a therapeutic focus on immuno-oncology and other diseases. Kiromic is in the process of developing a multi-indication allogeneic CAR-T cell therapy that exploits the natural potency of Gamma Delta T-cells to target solid cancers.
From its heritage as a cancer vaccine development company, Kiromic is focused on discovering, developing, and commercializing novel immuno-oncology applications through its robust product pipeline. The pipeline development is leveraged through the Company’s proprietary target discovery engine called "DIAMOND." Kiromic's DIAMOND is where big data science meets target identification to dramatically compress the man-years and billions of drug development dollars required to develop a live drug. The Company maintains offices in Houston, Texas. To learn more, visit www.kiromic.com and connect with us on Twitter and LinkedIn.
Forward-Looking Statements
This press release contains forward-looking statements that involve substantial risks and uncertainties. We make such forward-looking statements pursuant to the safe harbor provisions of the U.S. Private Securities Litigation Reform Act, Section 21E of the Securities Exchange Act of 1934, as amended, and other federal securities laws. All statements other than statements of historical facts are forward-looking statements. These statements relate to future events or to our future financial performance and involve known and unknown risks, uncertainties and other factors that may cause our actual results, levels of activity, performance or achievements to be materially different from any future results, levels of activity, performance or achievements expressed or implied by these forward-looking statements. Forward-looking statements include, but are not limited to, statements about: the use of KiroVax/BSK01 to treat metastatic solid malignancies including advanced metastatic pancreatic cancer; how clinical studies are indications of the Company’s clinical success in the cellular therapy arena; the impact of KiroVAX/BSK01 will have on the Company’s immunity cellular therapy pipeline; and the future role of KiroVax/BSK01 as an adjuvant in combination with the Company’s growing innate immunity cellular therapy platform.
In some cases, you can identify forward-looking statements by terms such as “we believe,” “may,” “in coming years,” “could,” “by,” “if,” “will,” “should,” “would,” “expect,” “plan,” “intend,” “anticipate,” “believe,” “estimate,” “predict,” “potential,” “project” or “continue” or the negative of these terms or other comparable terminology. These statements are only predictions. You should not place undue reliance on forward-looking statements because they involve known and unknown risks, uncertainties and other factors, which are, in some cases, beyond our control and which could materially affect results. Factors that may cause actual results to differ materially from current expectations include, among other things, those risks described in our filings with the Securities and Exchange Commission (SEC), including those discussed in our annual report on Form 10-K for the year ended December 31, 2020, in our quarterly reports on Form 10-Q for any subsequent quarterly periods, and elsewhere in this press release. If one or more of these risks or uncertainties occur, or if our underlying assumptions prove to be incorrect, actual events or results may vary significantly from those implied or projected by the forward-looking statements. No forward-looking statement is a guarantee of future performance. The forward-looking statements made in this report relate only to events or information as of the date on which the statements are made in this report. Except as expressly required by the federal securities laws, there is no undertaking to publicly update or revise any forward-looking statements, whether as a result of new information, future events, changed circumstances or any other reason.
View source version on businesswire.com: https://www.businesswire.com/news/home/20211025005273/en/
Contacts
Linda Phelan Dyson, MPH
Global Head, Investor Relations
Kiromic
ldyson@kiromic.com
973-986-5973





