– Rapid, deep, and durable reductions in high-sensitivity C-reactive protein (hs-CRP) through Day 90 achieved across all pacibekitug arms with high statistical significance as compared to placebo (p<0.0001 for all arms) –
– Pacibekitug becomes the first and only IL-6 inhibitor known to demonstrate deep hs-CRP reductions with quarterly dosing in a clinical trial, achieving >85% hs-CRP reductions from baseline in the 50 mg quarterly arm –
– Overall incidence rates of adverse events and serious adverse events in the pacibekitug groups were comparable to placebo through the data extract date –
– Results from TRANQUILITY support the advancement of pacibekitug into a potential Phase 3 cardiovascular outcomes trial in atherosclerotic cardiovascular disease and a planned Phase 2 proof-of-concept trial in abdominal aortic aneurysm –
– Conference call and webcast to be held today, May 20 at 8:30 a.m. ET –
NEW YORK, May 20, 2025 (GLOBE NEWSWIRE) -- Tourmaline Bio, Inc. (Tourmaline) (NASDAQ: TRML), a late-stage clinical biotechnology company developing transformative medicines to dramatically improve the lives of patients with life-altering immune and inflammatory diseases, today announced positive topline results from its ongoing Phase 2 TRANQUILITY trial evaluating quarterly and monthly subcutaneous dosing of pacibekitug in patients with elevated high-sensitivity C-reactive protein (hs-CRP), a biomarker associated with elevated cardiovascular risk, and chronic kidney disease (CKD). The TRANQUILITY trial is the starting point of Tourmaline’s clinical development program for pacibekitug for the potential treatment of atherosclerotic cardiovascular disease (ASCVD) and other cardiovascular diseases.
“We are thrilled with the data emerging from the TRANQUILITY trial and the remarkable consistency of signal across all endpoints evaluated. All active arms achieved rapid, deep, and durable reductions in hs-CRP, and overall incidence rates of adverse events and serious adverse events were comparable between pacibekitug and placebo. Importantly, this is the first time that pacibekitug or any IL-6 inhibitor has been tested in a clinical trial with quarterly dosing. It is well-recognized that less frequent administration has the potential to enhance patient adherence and ultimate clinical benefit,” said Sandeep Kulkarni, MD, Co-Founder and Chief Executive Officer of Tourmaline. “Given the strength of the data, we look forward to accelerating development of pacibekitug within atherosclerotic cardiovascular disease and abdominal aortic aneurysm. We anticipate sharing additional data from the TRANQUILITY trial at an upcoming medical conference.”
“There is strong evidence suggesting that elevated levels of high-sensitivity C-reaction protein (hs-CRP) are a predictor of increased cardiovascular risk, and quarterly subcutaneous administration of pacibekitug in the TRANQUILITY trial demonstrated large and significant reductions in hs-CRP levels through Day 90,” said Dr. Deepak L. Bhatt, Director of the Mount Sinai Fuster Heart Hospital, the Dr. Valentin Fuster Professor of Cardiovascular Medicine at the Icahn School of Medicine at Mount Sinai in New York, and Chair of Tourmaline’s Cardiovascular Scientific Advisory Board (for which he is compensated). “Based upon these results, pacibekitug merits testing in future Phase 3 trials for cardiovascular risk reduction.”
TRANQUILITY Topline Results:
TRANQUILITY Trial Design
TRANQUILITY (NCT06362759) is a multicenter, randomized, double-blind, placebo-controlled Phase 2 trial in patients with elevated hs-CRP and CKD stage 3 or 4. Participants were stratified by CKD stage and randomly assigned to receive subcutaneously administered pacibekitug 25 mg quarterly, 50 mg quarterly, or 15 mg monthly, or placebo, for a treatment period of 6 months. Participants are then followed for a period of 6 additional months.
The prespecified primary endpoint of the TRANQUILITY trial is median time-averaged percent change in hs-CRP through Day 90, adjusting for baseline hs-CRP levels. The key secondary endpoint is the percentage of participants achieving time-averaged hs-CRP below 2 mg/L through Day 90. Additional prespecified endpoints include the aforementioned hs-CRP endpoints at Day 90 (i.e., using single-timepoint analyses) as well as the percentage of participants achieving hs-CRP reductions of 50% or greater.
The prespecified primary analysis population includes participants who entered the study with a baseline hs-CRP of at least 1.9 mg/L (calculated as the average of Screening and Day 1 values), had at least one post-baseline hs-CRP assessment, and received all planned study drug doses during the primary evaluation period. The data in this analysis reflect a data extract date of April 23, 2025 from the ongoing trial.
TRANQUILITY Baseline Characteristics
A total of 143 participants were enrolled in the TRANQUILITY trial. Of this total, 126 participants comprised the primary analysis population. Baseline characteristics were generally balanced between groups.
Key baseline characteristics of the primary analysis population are as follows:
| Pacibekitug | ||||
| Placebo n=31 | 25 mg quarterly n=31 | 50 mg quarterly n=30 | 15 mg monthly n=34 | |
| Age, years | 72 (62, 77) | 73 (66, 76) | 70 (61, 78) | 65 (60, 73) |
| Female | 21 (68%) | 17 (55%) | 19 (63%) | 21 (62%) |
| ASCVD | 15 (48%) | 16 (52%) | 20 (67%) | 7 (21%) |
| Diabetes | 21 (68%) | 16 (52%) | 20 (67%) | 19 (56%) |
| Body-mass index, kg/m2 | 33.4 (29.7, 36.3) | 32.2 (28.1, 35.6) | 35.1 (30.7, 39.8) | 33.4 (30.2, 36.3) |
| eGFR, mL/min/1.73m2 | 43.5 (33.5, 53.0) | 37.5 (33.0, 47.0) | 48.0 (36.5, 56.5) | 42.5 (36.0, 57.0) |
| IL-6, pg/mL | 4.91 (3.32, 5.93) | 4.62 (2.87, 7.30) | 5.56 (3.58, 10.03) | 4.86 (2.39, 7.83) |
| hs-CRP, mg/L | 3.60 (2.70, 5.10) | 3.90 (2.35, 5.55) | 5.18 (3.60, 7.35) | 4.73 (3.80, 7.40) |
Data provided are median (interquartile range) or n (%). eGFR: estimated glomerular filtration rate.
TRANQUILITY Pharmacodynamic Data
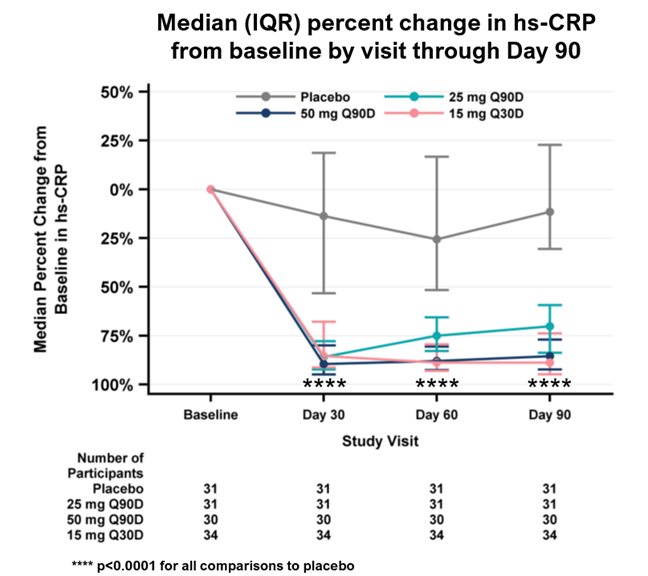
Key pharmacodynamic data for the primary analysis population are as follows (p<0.0001 for all comparisons to placebo):
| Pacibekitug | ||||
| Placebo n=31 | 25 mg quarterly n=31 | 50 mg quarterly n=30 | 15 mg monthly n=34 | |
| Change from baseline analyses: | ||||
| Median time-averaged percent reduction in hs-CRP through Day 90 (primary endpoint) | 15% | 75% | 86% | 85% |
| Median percent reduction in hs-CRP at Day 90 | 12% | 70% | 85% | 89% |
| Responder rate analyses: | ||||
| Percentage of participants achieving time-averaged hs-CRP < 2 mg/L through Day 90 | 26% | 81% | 80% | 88% |
| Percentage of participants achieving hs-CRP < 2 mg/L at Day 90 | 13% | 77% | 83% | 88% |
| Percentage of participants achieving time-averaged hs-CRP reduction >= 50% through Day 90 | 19% | 81% | 87% | 94% |
| Percentage of participants achieving hs-CRP reduction >= 50% at Day 90 | 16% | 81% | 90% | 91% |
Results of post-hoc sensitivity analyses of the primary endpoint in the intention-to-treat (ITT) population, i.e., all randomized participants, were highly consistent with the primary analyses above.
TRANQUILITY Safety Data
As of the data extract date, the cumulative incidence of any adverse event (AE) was 54% in the pooled pacibekitug group, compared with 56% in the placebo group. The most common AEs in the pooled pacibekitug group, defined as those occurring in at least 3 pacibekitug-treated participants, were urinary tract infection (4%), COVID-19 (3%), dizziness (3%), and viral upper respiratory tract infection (3%). The majority of AEs were mild or moderate in severity and were single occurrences in one participant each. The overall incidence of any serious adverse event (SAE) was 10% in the pooled pacibekitug group versus 11% in the placebo group. Incidence rates of infection (24% vs 22%) and serious infection (4% vs 3%) were similar in the pooled pacibekitug group compared with the placebo group. There was no increase in AE incidence with higher pacibekitug doses. There was one death, a fatal case of COVID-19, that occurred in the 25 mg quarterly arm. There were no Grade 2 or higher injection site reactions. There were no cases of confirmed Grade 3 or higher neutropenia and no cases of confirmed Grade 2 or higher thrombocytopenia. No clinically meaningful median percent changes in LDL cholesterol, triglycerides, or total cholesterol to HDL cholesterol ratio were observed in pacibekitug arms compared to placebo.
Cumulative safety data from the TRANQUILITY trial as of the data extract date are as follows:
| Pacibekitug | |||||
| Placebo n=36 | Pooled n=105 | 25 mg quarterly n=35 | 50 mg quarterly n=35 | 15 mg monthly n=35 | |
| AEs | 20 (56%) | 57 (54%) | 20 (57%) | 18 (51%) | 19 (54%) |
| SAEs | 4 (11%) | 10 (10%) | 6 (17%) | 2 (6%) | 2 (6%) |
| AEs leading to discontinuation | 0 | 2 (2%) | 0 | 1 (3%) | 1 (3%) |
| Infection | 8 (22%) | 25 (24%) | 10 (29%) | 9 (26%) | 6 (17%) |
| Serious infection | 1 (3%) | 4 (4%) | 4 (11%) | 0 | 0 |
| Death | 0 | 1 (1%)* | 1 (3%)* | 0 | 0 |
| Injection site reaction Grade 2+ | 0 | 0 | 0 | 0 | 0 |
| Neutropenia Grade 2 | 1 (3%) | 2 (2%) | 1 (3%) | 0 | 1 (3%) |
| Neutropenia Grade 3+ | 0 | 0 | 0 | 0 | 0 |
| Thrombocytopenia Grade 2+ | 0 | 0 | 0 | 0 | 0 |
Safety analysis population n=141.
*: Fatal case of COVID-19.
Future Development Plans for Pacibekitug in Cardiovascular Inflammation:
Tourmaline continues to make progress in advancing the clinical development strategy for pacibekitug in cardiovascular inflammation and assessing potential Phase 3 trial designs in patients with ASCVD. Tourmaline expects to provide further information on a potential Phase 3 cardiovascular outcomes trial in ASCVD later in 2025 following discussions with regulatory authorities.
Additionally, Tourmaline plans to initiate a Phase 2 proof-of-concept trial in patients with abdominal aortic aneurysm (AAA) in the second half of 2025. AAA is a common, serious vascular condition characterized by weakening and progressive enlargement of the abdominal aorta that can result in rupture and death. There is no approved medical therapy to slow or halt the growth of AAA. IL-6-driven inflammation is considered a critical mechanism promoting aneurysm expansion. The therapeutic potential of IL-6 inhibition for the treatment of AAA is supported by human genetic studies, epidemiological evidence, and mouse models of AAA.
“Both monthly and quarterly dosing of pacibekitug achieved significant, rapid, and sustained IL-6 pathway inhibition, as shown by robust reductions in hs-CRP in TRANQUILITY. We are excited to advance the development of pacibekitug to address significant unmet needs of patients with high-risk cardiovascular diseases driven by inflammation, including ASCVD and AAA,” said Emil deGoma, MD, Senior Vice President of Medical Research at Tourmaline.
Conference Call and Webcast Information:
Tourmaline will host a live conference call and webcast to discuss these results beginning at 8:30 a.m. ET today, May 20, 2025. Members of Tourmaline management will be joined by Dr. Deepak L. Bhatt, Director of the Mount Sinai Fuster Heart Hospital and the Dr. Valentin Fuster Professor of Cardiovascular Medicine at the Icahn School of Medicine at Mount Sinai in New York. Dr. Bhatt also serves as the Chair of Tourmaline’s Cardiovascular Scientific Advisory Board (for which he is compensated).
To register for this event, please click here or visit the Events and Presentations section of Tourmaline’s website. A replay of the event will be available on Tourmaline’s website following the event. It is recommended that participants register at least 15 minutes in advance of the event.
About Tourmaline Bio:
Tourmaline is a late-stage clinical biotechnology company driven by its mission to develop transformative medicines that dramatically improve the lives of patients with life-altering immune and inflammatory diseases. Tourmaline’s lead asset is pacibekitug. For more information about Tourmaline Bio and pacibekitug, please visit https://www.tourmalinebio.com or follow us on LinkedIn or X.
About Pacibekitug:
Pacibekitug is a long-acting, fully-human, anti-IL-6 monoclonal antibody with best-in-class potential and differentiated properties including a naturally long half-life, low immunogenicity, and high binding affinity to IL-6. Excluding ongoing trials, pacibekitug was previously studied in approximately 450 participants, including patients with autoimmune disorders, across six completed clinical trials. Tourmaline is currently developing pacibekitug in atherosclerotic cardiovascular disease (ASCVD) and thyroid eye disease (TED) as its first two indications, with plans to expand into abdominal aortic aneurysm (AAA) and additional diseases in the future.
Cautionary Note Regarding Forward-Looking Statements:
Any statements contained in this press release that do not describe historical facts may constitute forward-looking statements as that term is defined in the Private Securities Litigation Reform Act of 1995. These statements may be identified by words and phrases such as “believe,” “designed to,” “expect,” “may,” “plan,” “potential,” “will” and similar expressions, and are based on Tourmaline’s current beliefs and expectations. These forward-looking statements include expectations regarding the development and potential therapeutic benefits of pacibekitug, including the potential best-in-class profile of pacibekitug and the anticipated progression of pacibekitug into future clinical trials; the timing of initiation, progress and results of Tourmaline’s current and future clinical trials for pacibekitug, including the timing of a planned Phase 2 proof-of-concept clinical trial in patients with AAA and of Phase 3 clinical trial readiness in ASCVD, as well as reporting of data therefrom and additional details regarding the planning thereof; the timing of future announcements regarding Tourmaline’s development plans and the content of such announcements; the timing and likelihood of seeking regulatory approval for Tourmaline’s product candidates, including pacibekitug; the unmet need in Tourmaline’s target indications and the potential for pacibekitug to address such unmet need; and the timing and potential to expand pacibekitug into additional indications. These statements involve risks and uncertainties that could cause actual results to differ materially from those reflected in such statements.
Risks and uncertainties that may cause actual results to differ materially include additional safety events occurring after the data extract date of April 23, 2025, as participants complete their trial visits and follow-up throughout the course of the trial; a review of the full dataset (including the post-April 23, 2025 dataset), after the trial has completed, which may cause our analysis following completion of the trial to differ from the analysis as of the data extract date presented above; uncertainties inherent in the development of therapeutic product candidates, such as the risk that any one or more of Tourmaline’s current or future product candidates will not be successfully developed or commercialized; the risk of delay or cessation of any planned clinical trials of Tourmaline’s current or future product candidates; the risk that prior results, such as signals of safety, activity or durability of effect, observed from preclinical trials and clinical trials, will not be replicated or will not continue in ongoing or future studies or clinical trials involving Tourmaline’s current or future product candidates and/or current or future target indications; the risk that Tourmaline’s current or future product candidates or procedures in connection with the administration thereof will not have the safety or efficacy profile that Tourmaline anticipates; risks regarding the accuracy of Tourmaline’s estimates of expenses, capital requirements and needs for additional financing; changes in expected or existing competition; changes in the regulatory environment; the uncertainties and timing of the regulatory approval process; unexpected litigation or other disputes; the impacts of macroeconomic conditions on Tourmaline’s business, clinical trials and financial position; and other risks and uncertainties that are described in Tourmaline’s Quarterly Report on Form 10-Q filed with the U.S. Securities and Exchange Commission (“SEC”) on May 2, 2025 and other filings that Tourmaline makes with the SEC from time to time. Any forward-looking statements speak only as of the date of this press release and are based on information available to Tourmaline as of the date hereof, and Tourmaline assumes no obligation to, and does not intend to, update any forward-looking statements, whether as a result of new information, future events or otherwise.
Media Contact:
Scient PR
Sarah Mishek
SMishek@ScientPR.com
Investor Contact:
Meru Advisors
Lee M. Stern
lstern@meruadvisors.com
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/b1cc4485-af32-46c2-9fdb-144f4e1e573f






