Orthofix Medical Inc. (NASDAQ: OFIX), a leading global spine and orthopedics company, today announced the full U.S. commercial launch and successful completion of the first cases with the Galaxy Fixation Gemini™ system. The latest in the Galaxy product line, this stable external fixation system is provided in several sterile procedure kit configurations as a quick off-the-shelf solution for treating fractures that result from trauma in the lower and upper limbs.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20230920059213/en/
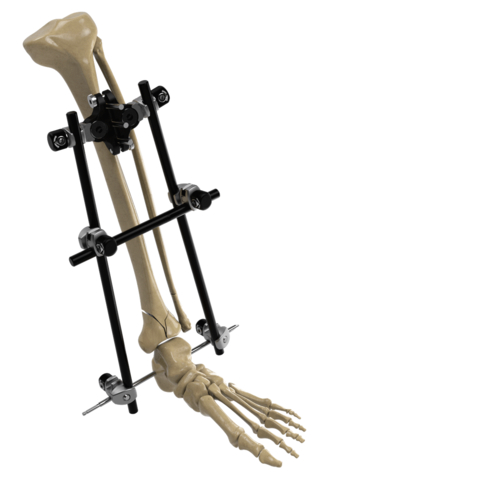
Image of the Galaxy Fixation Gemini System for treating fractures that result from trauma in the lower and upper limbs. (Photo: Business Wire)
“In trauma settings, it is critical that we respond quickly to manage the damage to the impacted limb,” said Dr. Evengy Dyskin, Clinical Assistant Professor of Orthopaedics, University of Buffalo, in Buffalo, NY. “The all-inclusive nature of the sterile kits eliminates spatial constraints, enabling application in a trauma bay or intensive care unit setting, which would not be possible with a traditional pin-to-bar system.”
Sterile kits eliminate the need for sterilization of trays which takes an average of 139 minutes1 to process and adds cost to healthcare facilities. Sterilization of trays also results in increased operating room (OR) delays and costs due to packaging, restocking and tray contamination. Average cost to a facility for OR delays related to tray contamination is $1,0811 which can be avoided through the use of prepacked sterile kits.
In particular, the Galaxy Fixation Gemini ankle kit is the only pin-to-bar system with specific clamps available in a sterile kit configuration giving surgeons more efficient solutions in lower extremity trauma situations where time is an important factor. The ankle kit, developed specifically to meet the needs of U.S. surgeons, includes a double multiscrew clamp to facilitate rapid insertion of tibial half-pins and is complemented by the foot support and first metatarsal sterile kits when a more robust construct is desired. It is estimated that ankle fractures occur in 187 per 100,000 persons per year in the U.S.2
“We are excited to expand our external fixation offerings in the U.S. for trauma through the introduction of the Galaxy Fixation Gemini System in several sterile packed configurations,” said Kim Elting, President of Global Orthopedics. “This streamlined pin-to-bar configuration is the latest example of our leadership in sterile kit solutions, which improve OR efficiencies and meaningfully decrease costs for the hospital and also for our business.”
Orthofix further expanded its sterile kit offerings with the global launch of the CalcFix Plus Calcaneal Minifixator™ System earlier this year. The CalcFix Plus represents the latest version of the original CalcFix Fixator device designed to treat calcaneal fractures using a more minimally invasive external fixation approach compared with internal fixation and includes updates to the design that reduce the number of operative steps to apply.
The Galaxy Fixation Gemini and the CalcFix Plus Systems add to Orthofix’s extensive portfolio of over 50 sterile kit procedural solutions available globally and demonstrate Orthofix’s continued investment in providing off-the-shelf solutions to meet the needs in the acute trauma, military and humanitarian crisis settings.
Those attending the AOFAS Annual Meeting in Louisville, KY Sept. 20-22 can learn more about the Galaxy Fixation Gemini System and the CalcFix Plus Calcaneal Minifixator System by visiting booth 613.
1 Ly JA, Wang WL, et al. Arch Bone Jt Surg, 2022 May; 10(5): 420-425.
2 Source (SmartTrack, 2023).
About Orthofix
On January 5, 2023, Orthofix and SeaSpine merged to form a leading global spine and orthopedics company with a comprehensive portfolio of biologics, innovative spinal hardware, bone growth therapies, specialized orthopedic solutions, and a leading surgical navigation system. Its products are distributed in approximately 68 countries worldwide.
The Company is headquartered in Lewisville, Texas and has primary offices in Carlsbad, CA, with a focus on spine and biologics product innovation and surgeon education, and Verona, Italy, with an emphasis on product innovation, production, and medical education for orthopedics. The combined Company’s global R&D, commercial and manufacturing footprint also includes facilities and offices in Irvine, CA, Toronto, Canada, Sunnyvale, CA, Wayne, PA, Olive Branch, MS, Maidenhead, UK, Munich, Germany, Paris, France and Sao Paulo, Brazil. A new name for the combined entity will be announced at a future date; the Company will continue to operate as Orthofix until said announcement.
Forward-Looking Statements
This news release may include forward-looking statements within the meaning of Section 21E of the Securities Exchange Act of 1934, as amended, and Section 27A of the Securities Act of 1933, as amended. In some cases, you can identify forward-looking statements by terminology such as “may,” “will,” “should,” “expects,” “plans,” “anticipates,” “believes,” “estimates,” “projects,” “intends,” “predicts,” “potential,” “continue” or other comparable terminology. Orthofix cautions you that statements included in this news release that are not a description of historical facts are forward-looking statements that are based on the Company’s current expectations and assumptions. Each forward-looking statement contained in this news release is subject to risks and uncertainties that could cause actual results to differ materially from those expressed or implied by such statement. Applicable risks and uncertainties include, among others: the ability of newly launched products to perform as designed and intended and to meet the needs of surgeons and patients, including as a result of the lack of robust clinical validation; and the risks identified under the heading “Risk Factors” in Orthofix Medical Inc.’s Annual Report on Form 10-K for the fiscal year ended December 31, 2022, which was filed with the Securities and Exchange Commission (SEC) on March 6, 2023. The Company’s public filings with the Securities and Exchange Commission are available at www.sec.gov. You are cautioned not to place undue reliance on forward-looking statements, which speak only as of the date when made. Orthofix does not intend to revise or update any forward-looking statement set forth in this news release to reflect events or circumstances arising after the date hereof, except as may be required by law.
View source version on businesswire.com: https://www.businesswire.com/news/home/20230920059213/en/
Contacts
Media Relations
Denise Landry
DeniseLandry@orthofix.com
214.937.2529
Investor Relations
Louisa Smith, Gilmartin Group
IR@orthofix.com





